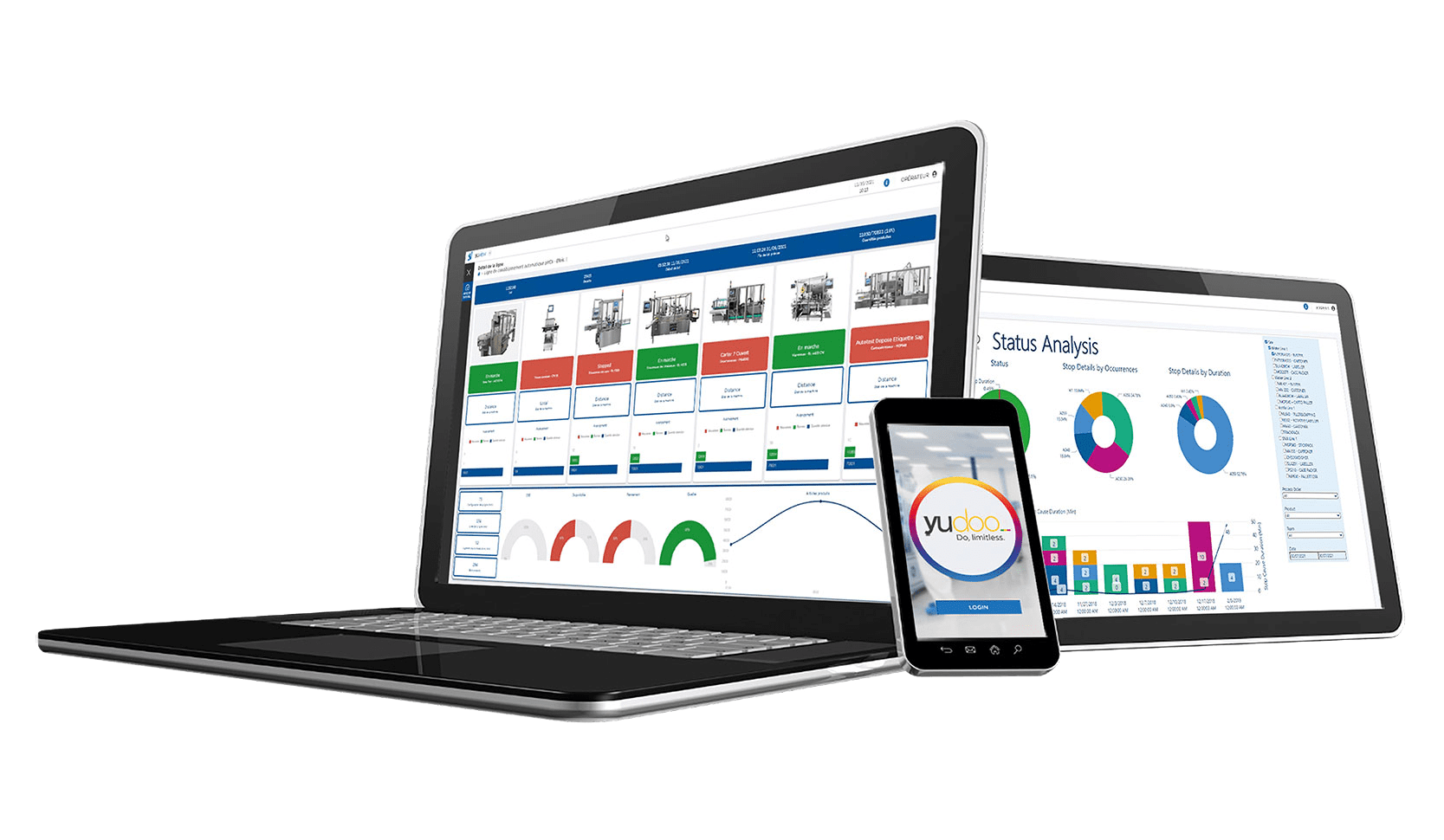
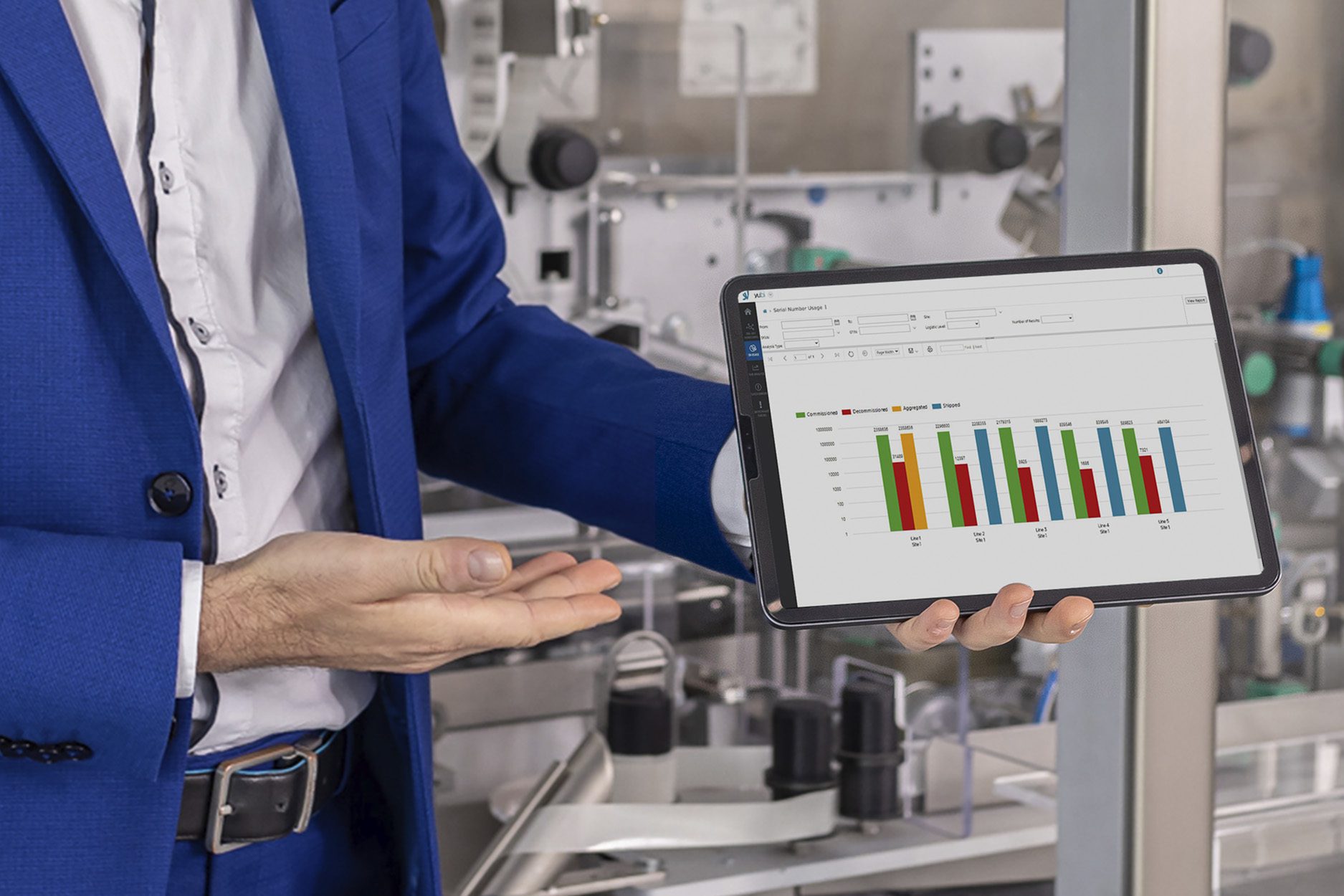
Serialization and aggregation operations involve a large amount of data. SEA Vision has created a solution to keep everything under control in a simple way through a dashboard that shows in real-time the provisioning and usage status of the serials.
Complete serialization monitoring
Management of one or multiple production sites
Increases efficiency
Reduces production stops and delays
Customizable
The manufacturing traceability solution for Track and trace monitoring allows to keep under control the large amount of data required for serialization and aggregation operations, clearly highlight all the information in very understandable and well-organized dashboards. This software enables a significant production efficiency improvement and allows to understand how to solve inefficiency problems and causes of production stops or delays.
With Track and Trace monitoring, it is possible to monitor the supply status of serial numbers for the production of the forecasted batches, detecting the exceeding and shortage quantities. The system can analyze production times and the supplying times of the serial numbers, drawing attention to communication criticalities. Moreover, all alarms and error messages are recorded.
With Track and trace monitoring, it is also possible to perform advanced business intelligence analysis on historicized data. To make an example, this powerful solution can allow the user to analyze and compare the production on one or more sites, to obtain deep and meaningful data insights.
Track and trace monitoring is fully compatible with any SEA Vision serialization solution; it is accessible anytime and anywhere via web browser from any mobile or desktop device. Access is controlled, and viewing permissions are customizable for each user or role. The installation has no impact on the existing infrastructure and doesn’t require line stops or revalidations.
The solution is specifically designed for the pharmaceutical market, and it is compliant with the industry regulations, with user access regulated by FDA 21 CFR part 11 and annex 11. All events are registered by the software in an audit trail file to keep track of all the security-relevant activities.
The continuous Software development follows Gamp 5, to always improve products quality and consistency.
Track and trace monitoring is natively integrated with Yudoo, the 4.0 pharmaceutical software suite for the full management of automation processes, digital quality, data analysis and for Track&Trace. A digital hub that is everywhere accessible through a secure-access web interface or dedicated devices.
A scalable and modular suite, including tools for automation (centralized management of production, workflows and timesheet), for the digital quality (creation of paperless systems, support systems for line clearance operations), for data analysis (analysis of production data, business intelligence dashboard, condition monitoring and predictive maintenance) and for Track and Trace (complete solutions for Level 3 and 4 of serialization, monitoring of serialization operations).
Yudoo offers multiple advantages such as the possibility to centralize production formats and data, avoiding duplicates and reducing work time. The suite can connect to existing company systems to retrieve updated data exactly from where they are.
SEA Vision provides an extensive product portfolio of natively integrated solutions. Vision, traceability and data management solutions for pharmaceutical packaging lines are developed with a common approach to obtain a seamless ecosystem.