Yudge is SEA Vision’s Level 4 Track & Trace solution, a contact point between the enterprise level and the Level 5 of National Regulatory Agencies. The Level 4 serialization solution developed by SEA Vision provides an extensive set of capabilities, to enable pharmaceutical companies to manage the different requirements for Track and Trace regulatories worldwide.
Suitable for MAH and CMO companies
Communication with regulatory agencies
Serial numbers importing or self-generation
Quick installation and integration with dedicated plug-ins
Modularity and Scalability
During the production, yudge self-generates or imports from L5 files or from other L4s the serial numbers that will be used and applied on each single product at line level. Serial number generation is entirely configurable through the use of re-usable rules and profiles.
Yudge connects itself to the regulatory agencies (e.g. EMVO, Markirovka) for serial numbers verification, data and event posting. All data relating to the serialization are safe: the serials, the events and related information are stored in a dedicated database.
Beyond production, yudge manages all the rework, shipment and distribution events related to serialized products.
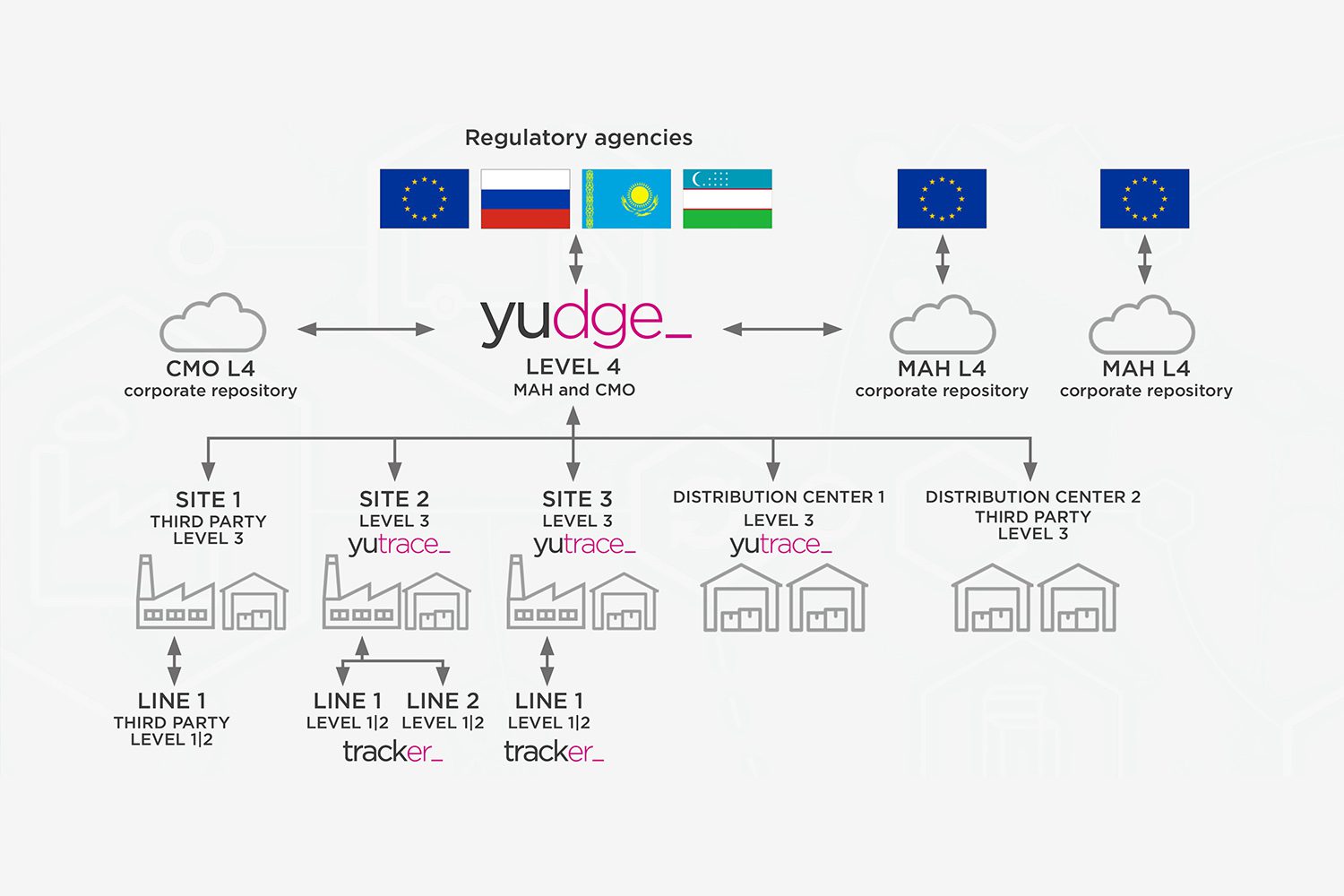
Yudge centralizes and manages the track and trace activities of every plant, line, warehouse and distribution center of a pharmaceutical company.
On cloud or on-premises? It’s up to you.
Yudge is flexible and can be installed on-cloud or on-premises adapting perfectly to every customer’s infrastructure and security needs.
The yudge modern web-based user interface is accessible from any kind of device: smartphone, notebook, desktop pc and wide smart monitor.


Nowadays, there is a global increase in regulations for the pharmaceutical industry that demands unique product identification solutions to contrast counterfeiting.
Yudge is a future-proof solution with integrable plug-ins that are developed for each specific market, to always keep up with track and trace regulations while safeguarding productivity with an easy validation process.
The system is specifically designed for the pharmaceutical market, and it is compliant with the industry regulations, with user access regulated by FDA 21 CFR part 11 and annex 11. All events are registered by the software in an audit trail file to keep track of all the security-relevant activities.
The continuous Software development follows Gamp 5, to always improve products quality and consistency.


Yudge is part of SEA Vision end-to-end track and trace solution that manages the entire cycle of pharmaceutical serialization and aggregation. Thanks to the native integration with Yudoo, SEA Vision 4.0 pharmaceutical software suite, the large amount of data collected can be analyzed and compared to obtain meaningful data insights.


