The Manual event input function provided by yudoo allows users to add information and comments about the reasons for idle events and manual stops, increasing the data collected and subsequently the report quality.
Complete information in addition to automated data collection
Information integration for a better understanding
More detailed reporting
Data set improved
No transfer of paper-based information
Manual stops
Knowing that most of the time equipment has been stopped due to manual reasons may not be enough when it comes to addressing efficiency issues. Manual stops need to be justified to identify precisely what actually stopped the machines and gain a deeper understanding of the more frequent stop causes.
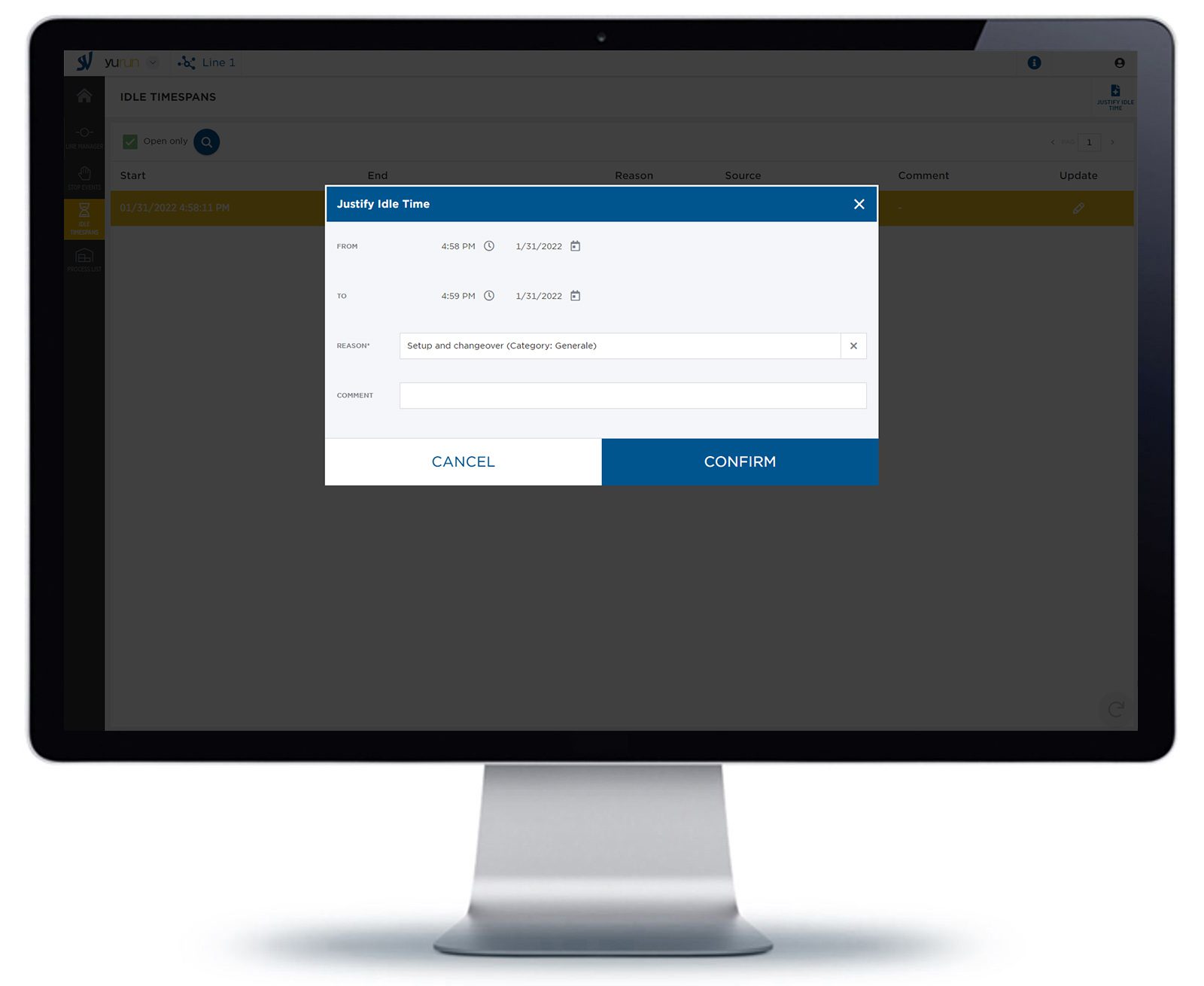
Idle events
Yudoo is able to detect idle events or underproduction events by analyzing their performance speed. But it is crucial to know why the events occurred. Thanks to the function idle events justification, it is easier to analyze idle reasons and address inefficiencies.
Comments and notes
Whenever there is the need to add a comment or a note for reporting or for quality reasons, yudoo offers the chance to insert additional information keeping full track of what happened during the production batch.
Features and compliance
The system is specifically designed for the pharmaceutical market, and it is compliant with the industry regulations, with user access regulated by FDA 21 CFR part 11 and annex 11. All events are registered by the software in an audit trail file to keep track of all the security-relevant activities.
The continuous Software development follows Gamp 5 regulation, to always improve products quality and consistency.
↓ Click below to discover all the other features of YUDOO FOR DIGITAL AUTOMATION ↓

SEA Vision provides an extensive product portfolio of natively integrated solutions. Vision, traceability and data management solutions for pharmaceutical packaging lines are developed with a common approach to obtain a seamless ecosystem.