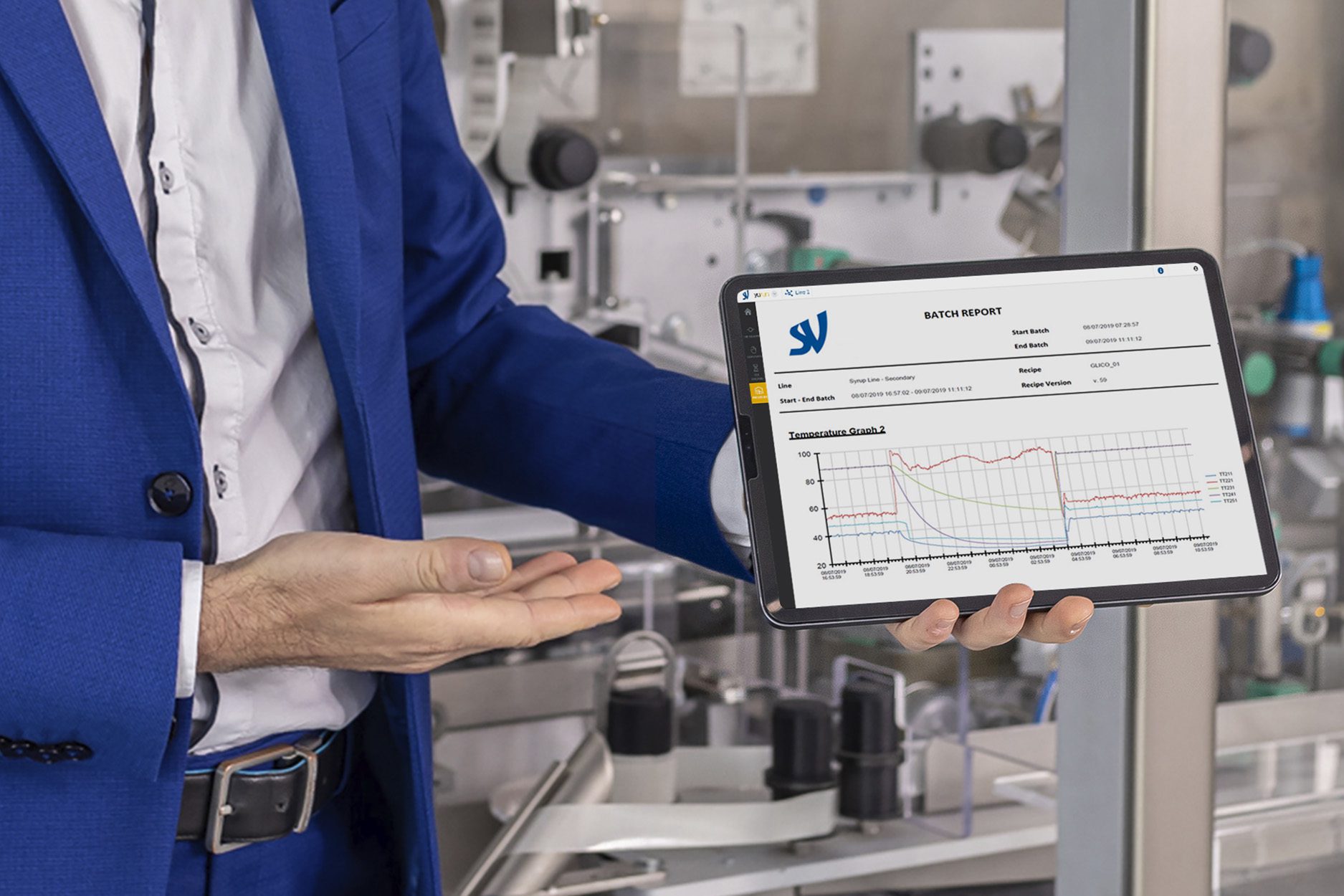
The Batch release analytical tool offered by yudoo, is an advanced manufacturing dashboard to boost batch release times.
Yudoo collects the data of each line asset (machine status, metrics, audit trail, process variables, temperatures, pressures) and presents a detailed automatic QA report with relevant GMP information to accelerate the batch release times.
Reduction of efforts to inspect different reports
Safety and compliance improvement
Reduction of the review time
Faster batch release
Fast time to market procedure
According to the different batch release processes (liquid forms, solids, primary or secondary packaging), it’s possible to pre-select the metrics to be extracted and spotlighted from the complete Audit Trail. They can be used to enrich the whole QA workflow further, by adding insights and the most important information on historical trends for analysis.
Features and compliance
The system is specifically designed for the pharmaceutical market, and it is compliant with the industry regulations, with user access regulated by FDA 21 CFR part 11 and annex 11. All events are registered by the software in an audit trail file to keep track of all the security-relevant activities.
The continuous Software development follows Gamp 5, to always improve products quality and consistency.
↓ Click below to discover all the other features of YUDOO FOR DIGITAL QUALITY ↓

With its extensive experience and wide range of product offerings, SEA Vision can provide an integrated and forward-thinking solution for all needs concerning vision, traceability and data management for pharmaceutical packaging lines.